Centre for Transfer of Biomedical Technologies has developed a system of effective technology transfer of research results into practice. On the basis of the experience that is being gained during the implementation of already running commercialization projects and through the good practice from foreign centers of excellence, the system is being constantly improved. For each particular case the individual paths and means are adapted on the case by case basis, though the basic scheme and the core process remains.
We have introduced a simple inexpensive system for very rapid commercialization - focusing on early communication with potential candidates, allowing flexible adaptation of the technology to the demand of potential customers, or entrusting the technology without major expense to one of our experienced foreign partners, accepting lower rate on returns or quickly terminanting commercialization activities, which will prevent us from wasting financial resources and time of the people - both ot the researchers and team members from Centre for Transfer of Biomedical Technologies.
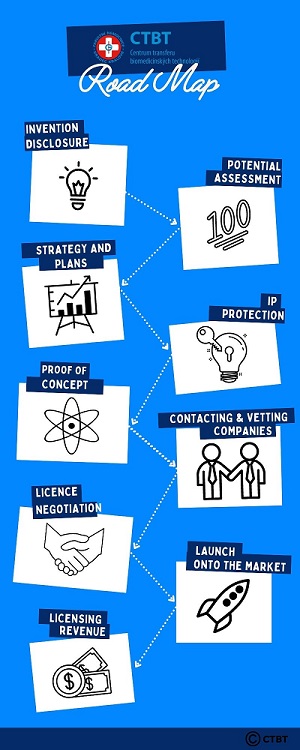
For the rough idea, each technology usually flows through these phases and decision points:
1. We receive invention disclosure - We put together with the inventors the most accurate idea of the invention, its possible uses, limitations and characteristics.
2. We perform or have an external expert perform technology outlook evaluation in terms of practical application possibilities and market opportunities, followed by a mandatory decision to be made: to accept / reject the technology for further commercialization efforts.
3. We prepare strategies and plans for commercialization, together with inventors we set control points, key activities and criteria for the success of each of the following phases.
4. We prepare and file the initial patent application, sometimes we do so at a later date, or we improve the application later on, or replace the initial with the better one, with one and only thing in mind - that the final offer to the potential candidates was the most powerfull possible.
5. We perform appropriate actions that increase the value of an invention for future licensees and we review and test useful technical properties, we acquire and refine prototypes (ie. Proof of Concept), we adapt the technology to the habits and needs of the market. We also often provide funding for this risky phase.
6. We actively offer the technology and search a suitable licencee or development partner.
7. We negotiate terms of the license with potential licensee to make trully mutually beneficial cooperation possible.
8. Licensee seek necessary registrations, officially tests the technology, so that he can place the final product on the market, while we provide appropriate support and technical advice.
9. After launch we monitor compliance with the license agreement, we receive returns from license and distribute the shares to inventors and institutions.