Filip, S1, Molnárová, V1, Novosadová, M2, Tichá, A3, Priester, P1, Švecová, D1
1 Department of Oncology and Radiotherapy, University Hospital Hradec Králové;
2 Department of Clinical Pharmacy, Hospital Pharmacy, University Hospital Hradec Králové;
3 Department of Clinical Biochemistry and Diagnostics, University Hospital Hradec Králové.
We are currently making significant progress with personalized strategies for cancer patients, especially in curative treatment. However, some patients under palliative care could be at a disadvantage if these strategies are limited to a single area of cancer treatment, thereby affecting the overall quality of the provided care. This grant proposal addresses this issue through the early identification of significant clinical risks under a personalized approach, which would include interventions in nutrition, pain management, polypharmacy, and compliance. This strategy has been implemented at a specialized workplace with a multidisciplinary team and with the collaboration of the palliative oncology care department. During the second year of the project (i.e., January 01 to November 30, 2021) a total of 335 patients (176 females and 160 males) were monitored and treated at the Palliative Cancer Care Outpatient Clinic (APOP). Of these patients, 205 had been recently taken into the department (100 females and 105 males). The study continued patient recruitment and evaluation in the areas of polypharmacy, compliance, and nutritional status. Regarding nutritional status and malnutrition risk assessment, the initial analysis included 53 patients, 29 females (54.7%) and 24 males (45.3%), with an average age of 51.7 years (42-92). It must be mentioned that the analysis was expanded to include other adipokine markers. Supportive nutritional care was evaluated through a sipping method. The results of the pilot study in the monitored patients confirmed that nutritional support, supplied through sipping, can prevent the loss of active tissue mass. This was evidenced by the significant improvement of the patients with nutritional intervention and dynamometry (e.g., weight, BMI, waist circumference, triceps skin folds, and body fat ratio). A total of 223 patients were enrolled in the polypharmacy and non-compliance risk monitoring evaluation, of which 113 were female (50.7%) and 110 were male (49.3%), with an average age of 71.25 years (21-94 years). The following tests were performed as part of the clinical trial: risk of pharmacodependence; risk factors in outpatients; and pharmacotherapy side effects. To date, the observed results from the cooperation format justify the inclusion of a clinical pharmacist as permanent member of the team. Further, mutual cooperation also improved the safety and efficacy of pharmacotherapy, thus promoting compliance and increasing the patient’s quality of life. The latter was considered as the third area of evaluation in regards to proper nutrition planning and nutritional intervention, as well as risk minimization and non-compliance. These goals were achieved during the second year of the study and the observed results have confirmed our preliminary assumptions.
Correspondence: Prof. MUDr. Stanislav Filip, Ph.D., DSc., Department of Oncology and Radiotherapy, University Hospital, Sokolská 548, 50005 Hradec Kralove, Czech Republic; stanislav.filip@fnhk.cz; tel. 495 834 618.
Published 14. 2. 2022 (BZ)
Researchers from University Hospital Hradec Králové came up with new diagnostic tool to measure gait quality and other neurological disorders. Wearable AI/ML computational unit with sensor to suggest immediate level of gait quality and disorder type and classification assessment has been developed.
Multiple sclerosis (MS) is the most common cause of neurological disability in young and middle-aged people. MS has a physical, psychological and financial impact on patients and their families. Up to 85 % of patients with MS identify gait disorders as a major problem. The ability to monitor the development of the disorder over time is highly valued diagnostic measure. Falling because of old age, neurological disorders, movement disorders and injuries can be predicted by the assessment of change in gait quality.
A set of wearable sensors and the first computing unit – assessing the overall gait quality in real time while walking, providing immediate feedback to the user or the physician. Subsequently, the second unit identifies several gait disorders, their extent and probable cause. In both cases, the evaluation is performed using machine-learning modules. Both approaches show relatively great robustness of the approach used and the relative simplicity of computer performance, especially in the near future. For general use the first step processing can warn patients or elderly people on the probability of falling.
This project is currently at TRL 3-4 stage of development. It is easy to use, yet reliable and robust diagnostics. It has automatic evaluation during which no expert is needed and on top of that, it functions as descision support for expert physician (neurologist). Our solution does not contain multiple joint sensors and expensive HW/SW, there is only one sensor unit that is well positioned. It can be used in real world, no laboratory assessment distortion.
Worldwide increasing incidence of MS was estimated to be 2.8 million people in 2020, in developed countries is double to triple incidence ‑ it might be due to lack of qualified diagnostics available in the developing countries ‑ the numbers are therefore supposedly undervalued. Can be helpful for many other neurological or movement diseases and elderly in general.
1. Non-MD/Diag. device – wearable “fall prediction”
2. Diagnostic – wearable – good/bad gait indicator
3. Diagnostic – wearable + mobile/tablet - gait disorder type and severity analyser and classifier
It is a opportunity for company/co-development partner to bring technology to the market as MD/Diagnostic and/or as a general public “indicative” device to warn about the risk of falling.
Published 20. 1. 2022 (BZ)
The Czech Science Fundation (GACR) has selected over 477 research projects to be funded starting next year including project from doc. Jan Korábečný from University Hospital Hradec Králové “The concept of rationally designed compounds for Alzheimer’s disease with a triple mechanism of action” and three others in the co-investigator role.
https://gacr.cz/en/czech-science-foundation-to-fund-nearly-500-new-research-projects/
Published 13. 1. 2022 (BZ)
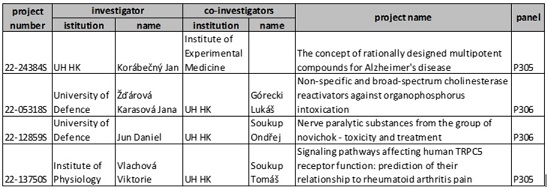
As part of the evaluation of internal grant competitions, the Commission for Science and Research recommended the implementation of 16 new research projects. At the same time, 8 applications for extension/continuation of projects implemented in 2021 were approved.
Congratulations!
Published 13. 1. 2022 (BZ)
At the beggining of this year, University Hospital Hradec Králové launched new Twitter accouned focused on news from research and development area. You can send your suggestions for new contributions to e-mail barbora.zoubkova@fnhk.cz. We will be happy for all your comments.
Published 13. 1. 2022 (BZ)
Prof. Kacerovský (Dpt. of Obstetrics and Gynecology, UH HK) and his research team succeeded in the prestigious Transfera Technology Day 2021 with the project for determination of the presence of specific bacteria in amniotic fluid collected by amniocentesis using multiplex RT-PCR test.
On October 21, 2021, the second year of the Transfera Technology Day event took place in the Prague National Technical Library. It aims to connect czech science with commercial sector and to create suitable conditions for mutual cooperation and provide valuable feedback on the commercial readiness of projects. A jury composed of representatives of investors, Technology Agency of the Czech Republic and companies evaluated the commercial potentil of research projects. A total of 34 projects from 14 institutions entered the competition, from which 12 finalists were finally selected.
34 projects, 12 finalists, 3 winners
The award for the third place went to the project presented by prof. Marian Kacerovský and Dr. Rudolf Kukla from University Hospital Hradec Králové for determination of the presence of specific bacteria in amniotic fluid collected by amniocentesis using multiplex RT-PCR test. Preterm Prelabour Rupture of Membranes (PPROM), during which amniotic sac rupture occurs (3 – 4% of pregnancies, i.e. 3 – 4 thousand pregnancies / year /Czech republic), is up to 1/3 complicated with the presence of bacteria in the amniotic fluid that lead to the development of intra-amniotic inflammation. Although this complication is usually asymptomatic (cannot be detected from standard blood tests of the mother), neonates from these pregnancies are at increased risk of developing neonatal sepsis and impaired psychomotor development. To treat this complication, it is necessary to know the cause of the inflammation so that the chosen antibiotic treatment is correctly targeted at specific bacteria from the beginning. This reduces the fetus exposure to the inflammatory environment of amniotic fluid and the risk of its damage due to infection is minimized. Currently, determining the presence of bacteria in amniotic fluid in patients with PPROM is very time-consuming and technically demanding. A combination of cultivation and non-cultivation laboratory methods is necessary. On top of that, results are available in days, which is already clinically irrelevant for the initiation of targeted antibiotic treatment.
Solution of prof. Kacerovský and his team is the determination of the presence of specific bacteria in amniotic fluid collected by amniocentesis using multiplex RT-PCR test allowing the detection of multiple bacteria from amniotic fluid at the same time. The test consists of 3 panels for detection of DNA of selected bacteria, which are the cause of 88% of all intra-amniotic inflammation. Panels contain specific primer sets, and the test results are interpreted by fluorescence method. Test would determine presence of specific bacteria in amniotic fluid within few hours with sensitivity of 90% and thus allows the timely initiation of targeted therapy leading to a reduction of the risk of possible complications for both mother and fetus.
The correctness of the direction of prof. Kacerovský research has also been verified during his internship at the Perinatology Research Branch in Detroit, where the team of prof. Romer has been dedicated to the problematics of preterm birth for couple of years. Also long-term and very successful cooperation with prof. Jacobsson from the University of Gothenburg in Sweden and prof. Cobo from the University of Barcelona in Spain helps the team of prof. Kacerovský keep the right direction of their research.
The jury appreciated both the social impact and need for this test, as well as the uniqueness of the clinical sample dataset, and awarded our project with 3rd place in this prestigious competition.
MD. Helena Linhartová from Centre for Transfer of Biomedical Technologies (CTBT) adds that it is the promotion of innovative technologies, such as at Transfera Technology Day, that helps research institutions look for partners from companies that might be interested in putting such product into practice.
CTBT already communicates with companies in the field of in vitro diagnostics, which could be interested in this unique test and would introduce it into the common use.
First place was given to the project from Palacký University in Olomouc for New special effervescent tablets for the treatment of contaminated water for both its uniqueness and in terms of readiness to enter the market. The winning technology can be used especially in case of natural disasters or industrial accidents. Tablets can be completely dissolved in contaminated water within 30 seconds, while being non-toxic and environmentally friendly. Second place was taken by the project connecting humanities and natural sciences of the J. Heyrovsky Institute of Physical Chemistry of the Academy of Science of the Czech Republic Microemulsions and gels for cleaning the surfaces of historical materials, which serves to remove their pollution.
Open doors to the winners
This year’s Transfera Technology Day was supported by CzechInvest agency and other partners such as Technology Agency of the Czech Republic, NEURON or vedavyzkum.cz portal. Success at this event should help projects find new partners or other financial resources and they will also receive an invitation to the CzechInvest Technology Incubator Program, which can financially support those projects as well.
Published 13. 1. 2022 (BZ)